Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kirishima, Akira; Kimura, Takaumi; Tochiyama, Osamu*; Yoshida, Zenko
Radiochimica Acta, 92(12), p.889 - 896, 2005/01
Times Cited Count:24 Percentile:81.07(Chemistry, Inorganic & Nuclear)no abstracts in English
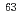 Ni migration through crushed rock media
Ni migration through crushed rock mediaTanaka, Tadao; Sakamoto, Yoshiaki; Mukai, Masayuki; Maeda, Toshikatsu; Nakayama, Shinichi
Radiochimica Acta, 92(9-11), p.725 - 729, 2004/12
Times Cited Count:1 Percentile:10.03(Chemistry, Inorganic & Nuclear)Migration experiments of 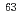 Ni for crushed rocks, granite and tuff, were performed under the coexistent condition with a humic acid and a fulvic acid of 0-30 mg/l in concentration, which are Nordic humic substances supplied from International Humic Substance Society. Migration experiments of Ni had been performed by a column system, to investigate migration behavior of Ni through a column packed crushed rock. The Ni concentration in the effluent passed through the column was corresponding to the fractional percentage of Ni complexing with humic substance in influent solution. This result suggests that the Ni complexing with humic substance in influent solution was flowed out from the column without any effective interactions with the rock media. The migration behavior of Ni could be expressed by a migration model taking account of the complexation kinetics of Ni with humic substance in the aqueous phase.
Ni for crushed rocks, granite and tuff, were performed under the coexistent condition with a humic acid and a fulvic acid of 0-30 mg/l in concentration, which are Nordic humic substances supplied from International Humic Substance Society. Migration experiments of Ni had been performed by a column system, to investigate migration behavior of Ni through a column packed crushed rock. The Ni concentration in the effluent passed through the column was corresponding to the fractional percentage of Ni complexing with humic substance in influent solution. This result suggests that the Ni complexing with humic substance in influent solution was flowed out from the column without any effective interactions with the rock media. The migration behavior of Ni could be expressed by a migration model taking account of the complexation kinetics of Ni with humic substance in the aqueous phase.
Yamaguchi, Tetsuji; Nakayama, Shinichi; Yoshida, Takahiro
Radiochimica Acta, 92(9-11), p.677 - 682, 2004/12
Times Cited Count:6 Percentile:40.72(Chemistry, Inorganic & Nuclear)Adsorption of actinides onto negatively charged mineral surfaces was investigated under conditions that actinides were predominantly present as anionic complex species: Th(CO )
)
 , Am(CO
, Am(CO )
)
 , Np(CO
, Np(CO )
) (OH)
(OH)
 , UO
, UO (OH)
(OH)
 , NpO
, NpO (OH)
(OH)
 , Sn(OH)
, Sn(OH)
 and Pb(OH)
and Pb(OH)
 . These solutions were left to stand for 2 days to confirm these elements stable in dissolved state, and then contacted with minerals,
. These solutions were left to stand for 2 days to confirm these elements stable in dissolved state, and then contacted with minerals,  -Al
-Al O
O or SiO
or SiO (AEROSIL, specific surface area: 10
(AEROSIL, specific surface area: 10 m
m kg
kg ). After desired contact time for 2 days or more, the solutions were ultra-filtered through 10
). After desired contact time for 2 days or more, the solutions were ultra-filtered through 10 -molecular-weight cutoff Millipore filters and the concentrations of the elements in the filtrates were determined. The sorption experiments were performed at room temperature (25
-molecular-weight cutoff Millipore filters and the concentrations of the elements in the filtrates were determined. The sorption experiments were performed at room temperature (25 C) under Ar. Distribution coefficients decreased with the increasing pH and with increasing carbonate concentrations. The monotonous decrease in the distribution coefficients in the investigated pH range suggests that the electrostatic repulsion was a dominant interaction between anionic complex species of actinides and negatively charged mineral surfaces.
C) under Ar. Distribution coefficients decreased with the increasing pH and with increasing carbonate concentrations. The monotonous decrease in the distribution coefficients in the investigated pH range suggests that the electrostatic repulsion was a dominant interaction between anionic complex species of actinides and negatively charged mineral surfaces.
Kirishima, Akira; Kimura, Takaumi; Nagaishi, Ryuji; Tochiyama, Osamu*
Radiochimica Acta, 92(9-11), p.705 - 710, 2004/12
Times Cited Count:28 Percentile:84.49(Chemistry, Inorganic & Nuclear)no abstracts in English
Yoshida, Takahiro; Ozaki, Takuo; Onuki, Toshihiko; Francis, A. J.*
Radiochimica Acta, 92(9-11), p.749 - 753, 2004/12
Times Cited Count:14 Percentile:66.09(Chemistry, Inorganic & Nuclear)Accumulation of Fe(III)-, Eu(III)-, Hf(IV)-, and Pu(IV)-desferrioxamine B (DFO) complexes by aerobic bacterium, 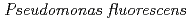 (ATCC 55241) was studied in order to elucidate stability of DFO complexes on biosorption. When Eu(III)-DFO complex was incubated with bacteria in 0.1 M Tris-HCl buffer (pH = 7.2), the metals were removed from solution, with no change in DFO concentration in solution. On the other hand, Fe(III)-, Hf(IV)-, and Pu(IV)-DFO complexes were not accumulated to bacteria. Metals with high charge to ionic radius ratio (Z/r) suppress the dissociation of complexes, leading small bacterial accumulation.
(ATCC 55241) was studied in order to elucidate stability of DFO complexes on biosorption. When Eu(III)-DFO complex was incubated with bacteria in 0.1 M Tris-HCl buffer (pH = 7.2), the metals were removed from solution, with no change in DFO concentration in solution. On the other hand, Fe(III)-, Hf(IV)-, and Pu(IV)-DFO complexes were not accumulated to bacteria. Metals with high charge to ionic radius ratio (Z/r) suppress the dissociation of complexes, leading small bacterial accumulation.
Sato, Haruo
Kokusai Kaigi Sanka Hokoku MIGRATION Kokusai Kaigi Migration'03: Dai-9-Kai Chichu Deno Akuchinido Oyobi Kaku Bunretsu Seiseibutsu No Kagaku Oyobi Iko Kyodo Ni Kansuru Kokusai Kaigi: Kaigi Hokoku, 0 Pages, 2003/00
None
 Concentration on Diffusion of C, Cl and I in Compacted Bentonite
Concentration on Diffusion of C, Cl and I in Compacted BentoniteIshidera, Takamitsu; Sato, Haruo; Miyamoto, Shinya
Migration 2003, PB-4, 145 Pages, 2003/00
None